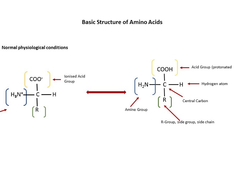
Amino Acids: Protein basics and more
Published: March 09, 2023
There are twenty amino acids required for the synthesis of thousands of different proteins in your body.
These amino acids belong to a group of more than 200 amino acids found in living organisms.
In addition to the 20 standard amino acids, this large group of amino acids also includes non-protein amino acids which have a variety of functions.
Essential amino acids
With respect to human growth and development, there are nine essential amino acids which the body does not synthesize.
These essential amino acids must be obtained from your diet. Non-essential amino acids are synthesised in your body.
A few amino acids are considered “conditionally” essential” in that their production depends on the availability of other amino acids and or amino acid components.
Limiting amino acids
A limiting amino acid is the essential amino acid found in the shortest supply in the food you consume relative to the amounts required for protein synthesis in your body.
All animal products (except gelatin) are considered sources of complete proteins as they contain all of the essential amino acids in sufficient amounts to meet the requirements of your body.
Consuming sufficient amounts of foods such as meat, fish, poultry, cheese, milk, yogurt and eggs will provide you with adequate amounts of all essential amino acids.
Plant sources of protein, such as legumes, grains and vegetables are mostly considered incomplete proteins.
Although these foods may contain all essential amino acids, they contain one or more limiting amino acid.
The limiting amino acids are: link to the full article to learn more.
References
1.
Gropper, S.S., Smith, J.L. & Groff, J.L. (2005). Advanced Nutrition and Human Metabolism (4thEd.). Belmont, CA: Thomson Wadsworth.
2.
Horton et al. (2002). Principles of Biochemistry (3rd Ed.). Upper Saddle River, NJ. Prentice Hall
3.
Whitney, E. & Rady Rolfes, S. (2005). Understanding Nutrition. Belmont, CA: Thomson Wadsworth